The Mysterious Function of a Neuronal Echo
/Image courtesy Wikimedia Commons
How do we visualize the brain in action? When one brain cell communicates with its partners, the message is fleeting. Action potentials, the basic currency of neuronal communication, are there and gone again in a millisecond burst of electricity. The transient nature of neuronal activity makes the job of a neuroscientist particularly tricky, like an anthropologist attempting to study a society that solely communicates via Snapchat.
An echo of neuronal activity
In 1990, Dr. Elizabeth Bullit published a study characterizing a new method that was gaining traction in the neuroscience community. The method takes advantage of a quirk of biology to grab a snapshot of neuronal activity. When a signal passes through an electrical circuit, each component simply receives and transmits the message, unperturbed by the flow of electricity. But neurons are different. Each time an electrical signal arrives at a cell, it sets off a cascade of events that changes the makeup of that cell—just the act of receiving a message changes how effectively the cell will receive and distribute future messages. When Dr. Bullit published her study, the main players in this cascade were poised to become rockstars of the neuroscience world.
The players are proteins known as immediate early gene transcription factors or IEGs, and two key attributes make them ideal for capturing neuronal activity. When a signal arrives at the cell, IEGs are responsible for turning genes on or off to prepare for the next message. This means that they are reliably present in neurons that are in the process of sending and receiving messages. Not only that, but unlike the electrical signal, they stick around for up to a couple hours. These factors make IEGs a kind of echo of neuronal activity, reverberating through the cells long after the message itself has faded. By looking to see which neurons express IEGs, we can see which cells have been talking to one another recently and where in the brain they reside, allowing us to draw connections between our moment-to-moment experiences and the activity of our brains.
Exploring the mystery of memory
Figure 1: Clusters of neurons act as unique stamps for memories
Small groups of neurons become active together during a particular memory, like when this mouse receives a footshock (A, yellow). Researchers can use IEGs to label these neurons and reactivate them, causing the brain to “remember” it’s prior experiences (B). In this case, when one group of neurons is turned on, the mouse recalls the footshock and freezes in fear.
With this new tool, scientists have begun to unravel one of the deepest mysteries of the human brain—how it learns, stores, and recalls memories. Before the 90’s, capturing a memory was a nearly impossible task. How could you connect a fleeting burst of electrical activity to a particular experience? IEGs would serve as that connection. Using IEGs, scientists could search for neuronal echoes left behind in the hippocampus, a brain region famous for its role in forming new memories. Their surprising finding was that during a new experience, most hippocampal neurons were silent, with only small groups of neurons actively chatting with one another. They hypothesized that these groups of cells might be the brain’s way of representing that particular experience.
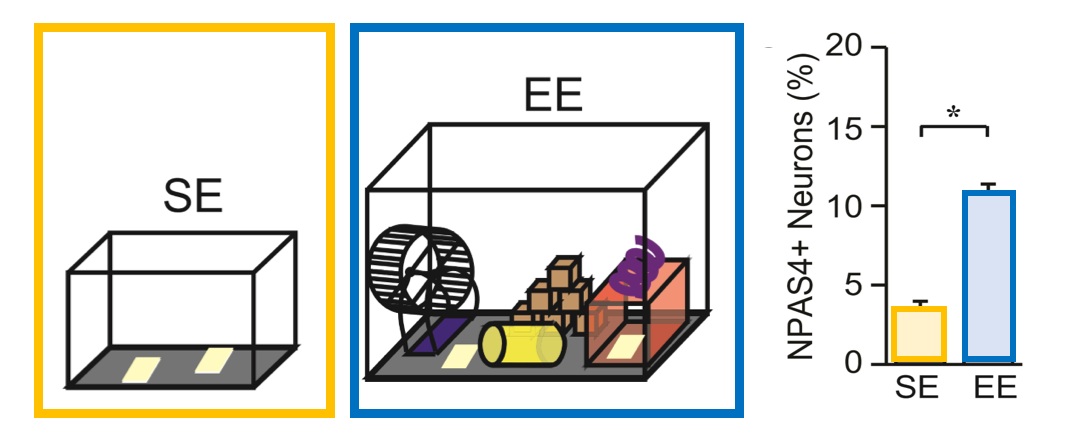
Figure 2: When mice explore rich sensory environments, they express more NPAS4
Mice exploring a rich sensory environment, with lots of new toys, form stronger connections between groups of neurons as they learn and build new memories. Active neurons in this enriched environment express more of the immediate early gene transcription factor, NPAS4. (Adapted from Hartzell et al, 2018)
But were these chatty groups of neurons really important to memory at all? Dr. Susumu Tonegawa and his colleagues at MIT investigate this question using sophisticated techniques that leverage IEGs to label, turn on, or silence active cells. To explore the role that the groups of neurons played in memory, the MIT scientists placed mice into different rooms so that they could form a memory of each. One of the rooms was harmless, but in the other the mice received a light shock to their foot. Using IEGs to label talkative neurons in each room, the scientists found that different experiences activated different groups of cells; each cluster of cells seemed to be a stamp for that particular experience. Even more exciting, when they turned on the group of neurons from the room with the shock, the mice froze in fear. In other words, reactivating this particular group of neurons caused the brain to “remember” its prior experience (Fig. 1).
Dr. Tonegawa’s studies tell an intriguing story in which hippocampal cells engage in a complex choreography of electrical signals to build memories. Certain details will cause one group to clamor, passing neural phrases from one cell to the next and awaking the memory of an electric foot shock. Other details will leave that group silent, with a different cluster of cells taking up the chant. Which cell is a member of which group, and when they exchange messages or remain silent, depends on the strength of the connections between each cell and its partners. But who choreographs these complex relationships? In a cinematic twist of fate, new research suggests that the very IEGs that have been so useful for studying memory might themselves be the answer.
Figure 3: NPAS4 helps strengthen connections between cells
NPAS4 is important for forging stronger connections between particularly active neurons during learning and memory formation. Dr. Bloodgood’s group measured the strength of connections between neurons (A) and used recording electrodes to determine the size of the messages passing between them (B). Normally, an enriched environment enhances the strength of messages and connections between neurons (blue boxes), as mice form new memories of the toys. Neurons without NPAS4 (green bar and traces) don’t form these extra strong connections anymore. (Adapted from Hartzell et al, 2018)
The biological purpose of a useful tool
IEGs have played a pivotal part in nearly thirty years of neuroscience research, but until very recently we had almost no idea how the brain uses them or why it would produce them at all. Why do they show up in particularly talkative neurons? One possibility is that they are involved in long-term changes associated with these neurons. For example, they could make an active neuron more likely to fire again in the future. Or maybe they help to forge stronger connections between that neuron and its partners, so that when one member of the group starts chatting, the rest will join in. Could it be that IEGs underly the construction of new memories in the hippocampus? Dr. Brenda Bloodgood’s lab at the University of San Diego is exploring exactly this question by looking at the role one particular IEG plays in the mouse hippocampus.
In a series of elegant experiments, Dr. Bloodgood and her colleagues have discovered that one particular IEG, NPAS4 (pronounced en-pass 4), adjusts hippocampal connections with striking precision. When mice played in a rich sensory environment, with plenty of toys to explore, their neurons became more active and began to express NPAS4 (fig. 2). These kinds of rich sensory experiences result in stronger connections between particular groups of neurons as the mouse learns about its world and builds new memories. Tantalizingly, when the scientists prevented hippocampal neurons from expressing NPAS4, the groups of cells no longer forged these connections (Fig. 3). This provides a clue as to what IEGs are doing in active neurons; NPAS4 might serve an important role in strengthening the connections between specific hippocampal cells. In doing so, NPAS4 could shift the delicate choreography of the groups of cells, resulting in a stronger response to some sensory details, while at the same time reducing responses to other details.
To be continued…
When IEGs first emerged on the stage in the late 80’s, nobody could have predicted their pivotal role as a tool for exploring memory formation. Dr. Bloodgood’s research marks the next act for these proteins, in which the perspective shifts away from using IEGs as a tool and instead focuses on their natural biological role in memory formation. While this preliminary research is an exciting first look at how one IEG choreographs hippocampal circuits, the full impact of these changes has yet to be discovered. Intriguingly, new work in the Bloodgood Lab suggests that this protein may enhance the neuronal response to particular locations in an environment. Perhaps NPAS4 helps the brain develop strong memories of salient places in just a short time. The story of IEGs in neuroscience is ongoing—we are only just beginning to uncover the true purpose of these reverberating echoes of neuronal activity.
Edited by Kristin Muench
References:
Bullitt, Elizabeth. "Expression of c‐fos‐like protein as a marker for neuronal activity following noxious stimulation in the rat." Journal of Comparative Neurology 296.4 (1990): 517-530.
Tonegawa, Susumu, et al. "Memory engram cells have come of age." Neuron 87.5 (2015): 918-931.
Liu, Xu, Steve Ramirez, and Susumu Tonegawa. "Inception of a false memory by optogenetic manipulation of a hippocampal memory engram." Philosophical Transactions of the Royal Society B: Biological Sciences 369.1633 (2014): 20130142.
Bloodgood, Brenda L., et al. "The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition." Nature 503.7474 (2013): 121.
Hartzell, Andrea L., et al. "NPAS4 recruits CCK basket cell synapses and enhances cannabinoid-sensitive inhibition in the mouse hippocampus." eLife 7 (2018): e35927.
Payne et al. (SfN 2018, Monday AM, poster 331.12).